The above image is just an animated gif file for those who cannot install the required Java software to have an idea about the simulation. Click the above button or this link to launch this interactive activity.
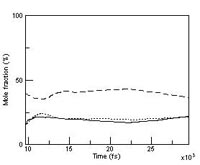
| Chemical reactions are in dynamical equilibria, which means the individual elementary reactions never cease. But at the macroscopic level, the reaction seems to have come to a stop. How does this happen? This molecular dynamics simulation shows you how. What is interesting about this simulation is that the chemical equilibrium is an emergent behavior from the molecular dynamics simulation. |


The above image is just an animated gif file for those who cannot install the required Java software to have an idea about the simulation. Click the above button or this link to launch this interactive activity. |
| System requirements: You must have Java Version 5 or higher in order to run this program. Please go to java.com to get the latest Java software, if you are not sure. |
| Failed? Check out the FAQ for troubleshooting. |