The above image is just an animated gif file. Click the above button or this link to launch this interactive activity.
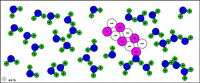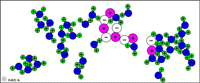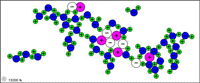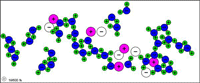
| Table salt (sodium chloride) dissolves in water because the attractions to Na+ and Cl- from water molecules become strong enough to break the ionic bonds. |

The above image is just an animated gif file. Click the above button or this link to launch this interactive activity. |
| System requirements: You must have Java Version 5 or higher in order to run this program. Please go to java.com to get the latest Java software, if you are not sure. |
| Failed? Check out the FAQ for troubleshooting. |